At BNZ Research Network LLC, we understand what sponsors and CROs value most: speed, reliability, quality data, and site accountability. Our network spans over 60 pre-vetted, high-enrolling clinical research sites across the U.S, covering Phase I to IV trials across nearly all therapeutic areas.
We don’t just connect you with sites — we match your protocol with the right investigators, manage the entire startup phase, and handle contract and budget execution with unmatched responsiveness.
Our diverse network of clinical sites spans multiple geographic locations and specialties, giving you access to the ideal setting for your study.
Each site is equipped with state-of-the-art infrastructure and supported by highly experienced physicians, skilled Principal Investigators (PIs), and qualified research staff.
Our sites strictly adhere to local and international regulatory requirements, ensuring your study runs smoothly and meets all compliance standards.
A Confidential Disclosure Agreement (CDA) is always in place to safeguard your study’s confidentiality and data security.
We conduct comprehensive feasibility checks to match your study requirements with the most suitable sites, ensuring you start with the ideal site for optimal outcomes.
Our team specializes in accelerating the start-up process, handling all start-up documentation, timely submissions, scheduling Site Selection Visit and much more with efficiency to get your study up and running without delays.
We handle all aspects of
budget negotiation, ensuring a single, transparent agreement tailored to your financial and operational needs.
We streamline the agreement process, including start-up agreements, Site/Investigator contracts, Clinical Trial Agreements (CTA), and other essential documentation, to eliminate delays and complexity.
Centralized communication — you deal with one experienced team rather than 60+ individual sites.
We provide transparent updates, timelines, and progress tracking throughout the startup phase.
We handle all visit coordination, aligning site schedules with your availability and ensuring all pre-visit documents are in place.
Our team prepares sites in advance and assigns a dedicated liaison to streamline communication and keep the process smooth and efficient.

Our network features board-certified principal investigators with extensive research expertise and a demonstrated history of success. Committed to excellence, they ensure rapid responsiveness, consistently meet enrollment goals, and deliver high-quality data on time.

Each site is supported by a trained and committed research team including blinding specialists, clinical research coordinators, support personnel, and regulatory experts —all working together to efficiently handle queries, documentation, and patient care with speed and precision

Our sites are equipped with state-of-the-art trial conduction infrastructure, including fully compliant pharmacy, precision-calibrated laboratories, advanced equipment, and dedicated research spaces — designed to meet protocol demands with ease and accuracy.
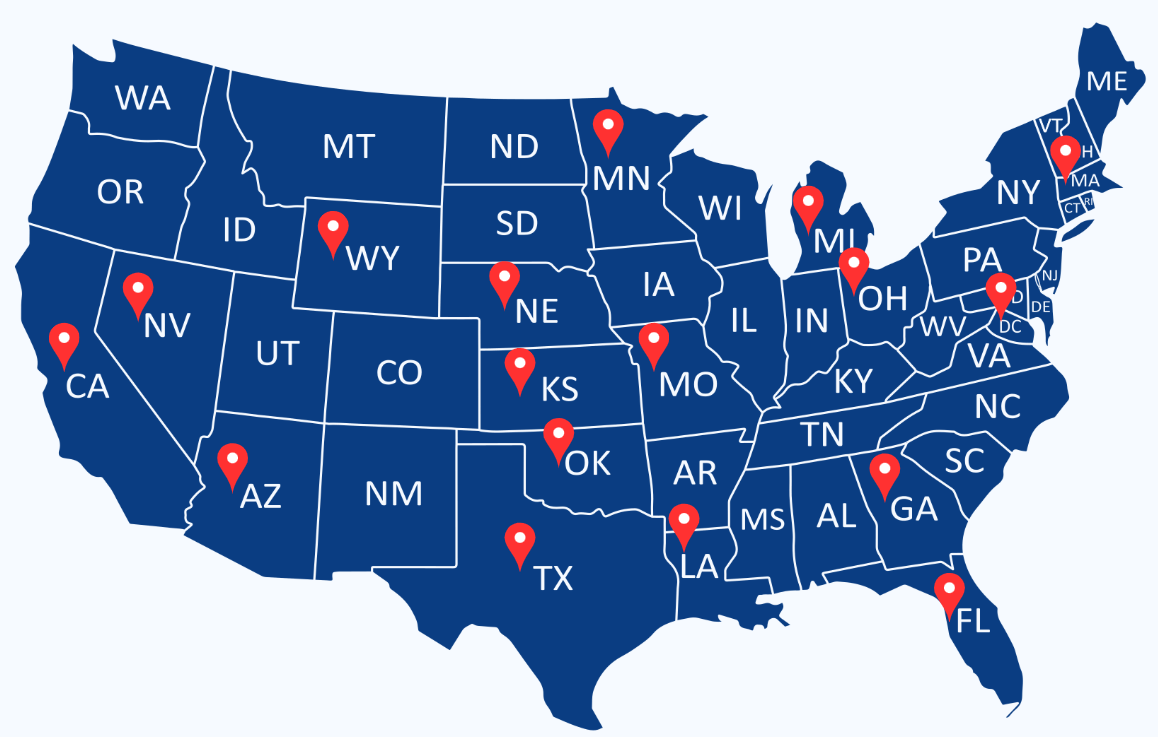
At BNZ Research Network LLC, our footprint spans across the USA, offering sponsors and CROs broad geographic reach with consistent quality.
We have active, pre-vetted research sites — each equipped with experienced PIs, dedicated staff, and proven trial performance.
Our Network is growing — fast.
If you’re looking for a reliable partner to de-risk your startup process and maximize trial efficiency, BNZ is ready to deliver. Let us show you how our sites and systems are built for speed — without compromising on quality.

Challenge: A global sponsor experienced poor enrollment and site dropout during a large-scale oncology trial across the U.S.
Solution: BNZ provided rapid rescue site support. Within 15 business days, we:
Identified and matched 2 high-performing oncology sites
Completed feasibility and document submissions
Negotiated contracts and budgets
Scheduled and completed PSVs
Challenge: A CRO managing a rare disease study needed a diverse site with an engaged PI in a specific region.
Solution:
BNZ identified a matching site within 4 days
PI had experience with rare diseases and a history of clean audits
Study materials submitted within a week
Challenge: A sponsor needed to activate multiple rheumatology sites across different U.S. states, each with varying timelines.
Solution: BNZ successfully met the geographic coverage requirement while maintaining high site and investigator quality, we:
provided 4 geographically distributed, experienced endocrinology sites & board certified PIs
Rapid feasibility submission and streamline approval
Customized a single budget for all sites
Challenge:
A CRO managing a Phase III psychiatry trial needed to activate 5 geographically distributed sites, each with unique startup requirements. Previously overwhelmed by fragmented communication, inconsistent timelines, and delays in feasibility and contracting.
Solution:
BNZ stepped in as the single point of contact for all 5 sites, Our team:
Submitted all 5 sites together in a single batch for feasibility and initial documentation
Consolidated FQ responses and site profiles
Scheduled and aligned PSV/SSV visits through one dedicated project lead
Handled contract and budget negotiations centrally, ensuring fast and hassle-free CTA execution for all sites
Get in touch at contact@bnzresearchnetwork.com
or visit our Contact page to start the conversation.
